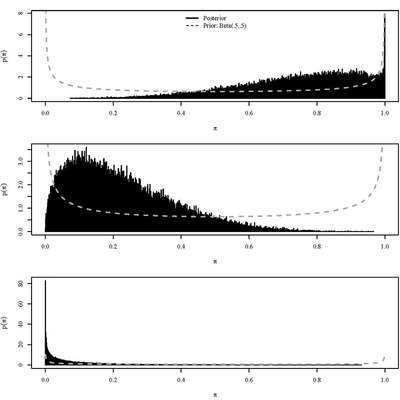
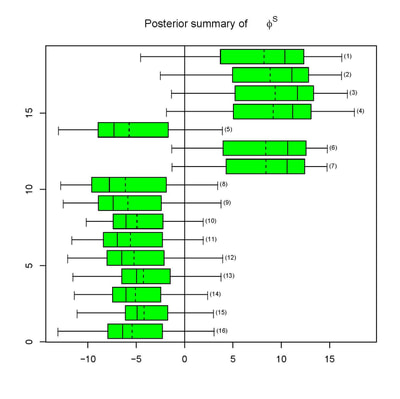
We offer statistical design and analysis services to clients in biomedical science, with an emphasis on hierarchical Bayesian methods. These are meta-analytic methods that enable efficient combination of information from disparate sources, including historical data. This enables significant flexibility; for instance:
Our list of past clients is long, and contains some of the biggest names in the pharmaceutical and medical device industries; recent consultees include Sanofi, Kognate, ExploreData, Synergy, and Boston Scientific. Do you need to design an adaptive clinical trial, combine disparate data sources, or fit a complex model in a drug or medical device setting? Counterpoint is for you.
We also have the experience to serve as statistician for clinical trial Data and Safety Monitoring Boards (DSMBs) and Institutional Review Boards (IRBs).
- Trials that can adapt based on the data collected so far, in order to stop early for efficacy, safety, or futility, or to change the randomization ratio
- Trials that utilize data on historical controls where that is justified, thus saving control patients in the current study
Our list of past clients is long, and contains some of the biggest names in the pharmaceutical and medical device industries; recent consultees include Sanofi, Kognate, ExploreData, Synergy, and Boston Scientific. Do you need to design an adaptive clinical trial, combine disparate data sources, or fit a complex model in a drug or medical device setting? Counterpoint is for you.
We also have the experience to serve as statistician for clinical trial Data and Safety Monitoring Boards (DSMBs) and Institutional Review Boards (IRBs).
|
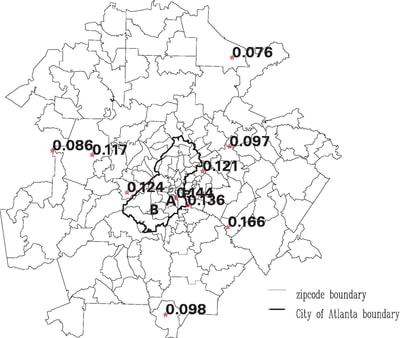
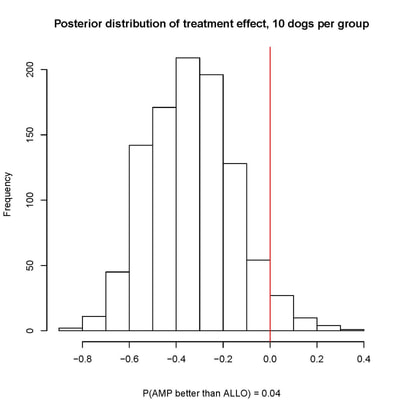
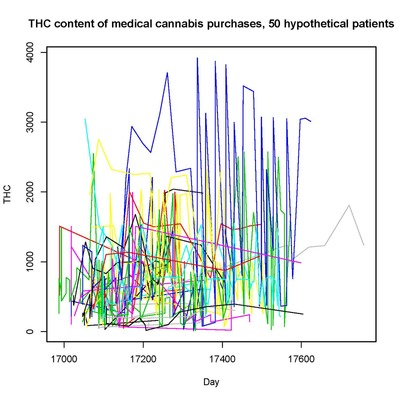
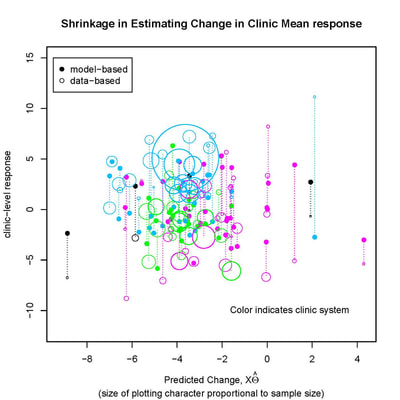
Our recent past work has covered a broad range of problems in biostatistics and clinical trials, including :
|
Our range of application areas in pharmaceutical and medical device research is also broad :
|
|
Copyright 2024 Counterpoint Statistical Consulting, LLC
|